Probing Periodontal Disease
NIDCR celebrates 75 years of progress to understand a common condition
During NIDCR’s 75th anniversary, a recurring theme at talks and seminars can be summarized in the immortal words of William Shakespeare: “What’s past is prologue.” The stage is set for a golden age of discovery in oral and craniofacial research. An unprecedented convergence of science and technology advances will translate these discoveries into greater diagnostic precision, enabling clinicians and scientists to pinpoint the underlying biology driving the diseases of the dental, oral, and craniofacial complex. Such biological information will allow practitioners to shift from one-size-fits-all therapies to treatments tailored to patients’ individual needs.
This future is now being crafted, discovery by compelling discovery, in laboratories around the world. An excellent case in point is NIDCR’s support of research to improve care for one of the most common conditions in the world: periodontal disease. In the United States, it affects an estimated 47% of adults over age 30 and 70% of adults over age 65.
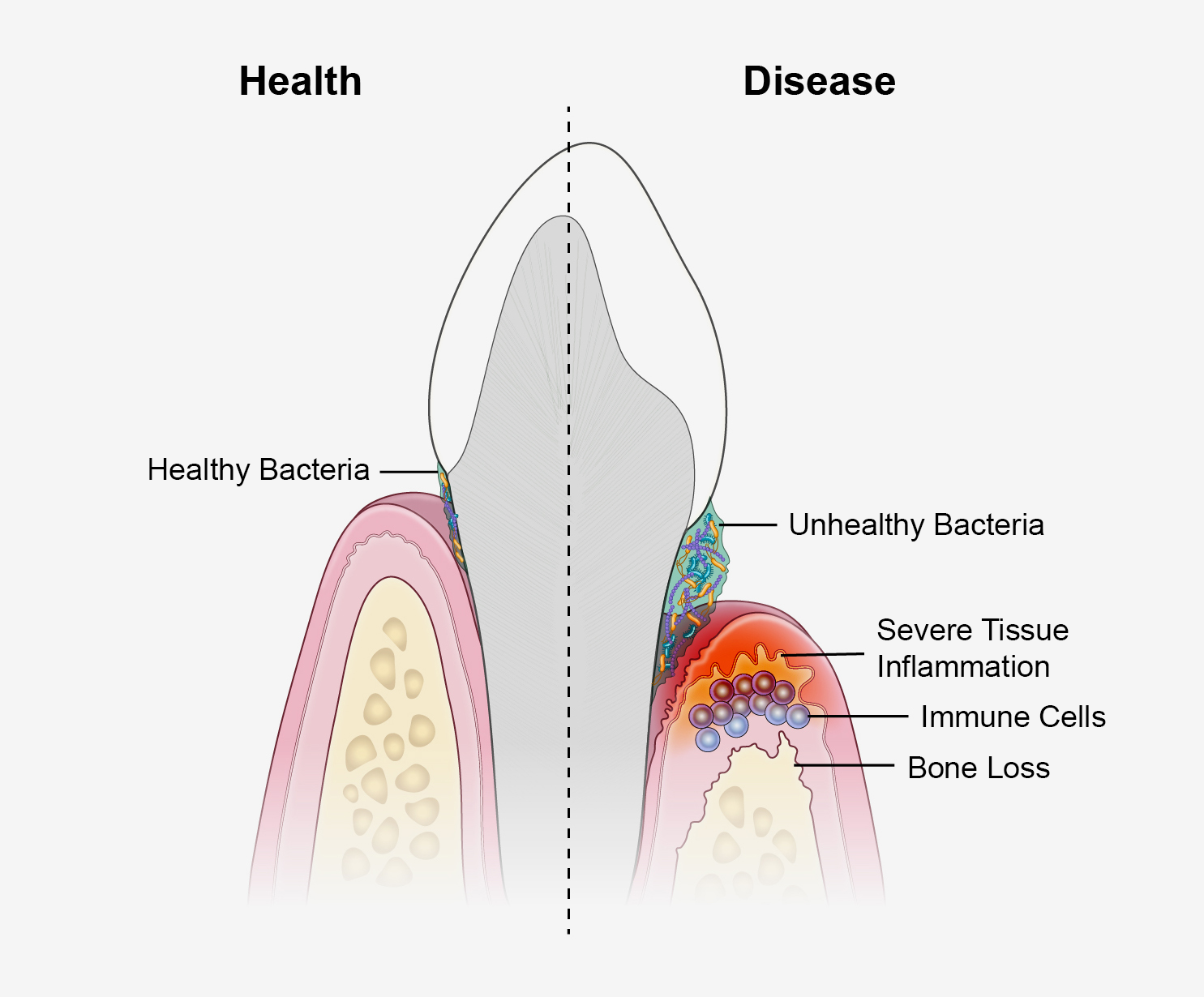
Many of us know it well. Most notice periodontal disease as a mild oral infection that causes reddening and mild bleeding of the gumline around a tooth, called gingivitis. With better oral hygiene and vigilance, the redness typically goes away. But for an estimated 9% of U.S. adults, the infection triggers prolonged bouts of inflammation, or periodontitis, that can progressively damage the supportive tissues and bone that hold a tooth in place. This can cause pain, difficulty chewing, and tooth loss.
Thanks to better treatments and enhanced public awareness of periodontal disease, significant progress has been made to reduce its impact on people’s lives. But there’s still plenty of work to do. Periodontitis stubbornly remains a leading cause of tooth loss, and it’s expected to become more common as baby boomers in the U.S. age. The condition also disproportionately affects non-Hispanic Black, Mexican-American, and lower-income populations. New treatments are greatly needed that leverage the latest technological advances and begin to account for the disease’s underlying biology.
“Better, more accessible treatments are tantalizingly within reach of the science,” said NIDCR director Rena D’Souza, D.D.S., Ph.D., M.S. “It’s never wise to place a timeframe on science. However, NIDCR has made strategic investments to accelerate the discovery and translation processes. We’re working to help all people keep their teeth for a lifetime by translating what we know into what we do.”
Firm Foundation
Over the past 75 years, research on periodontal disease has traversed many theories on its causation, mastered many newfangled technologies to expand its capabilities, and ultimately followed the data. Today, those data have established a firm research foundation and feature three main pillars upon which to build clinical progress:
Understanding the Oral Microbiome: The sticky, mat-like microbial communities found throughout nature and in many parts of the human body answer to many names. That includes plaque, or preferably, biofilm. In the mouth, biofilms form on the surfaces of our teeth and pack in hundreds of distinct bacterial species, plus fungi, viruses, and other organisms. All cooperate to help each other adapt to the constant fluctuations in food, mechanical stress, acidity, and so on that occur in the mouth over the course of a normal day.
In recent years, researchers have embraced another more macro term: microbiome. It highlights biofilms as unique ecological systems in which the sum of their microbial parts, including their genes, is greater than the effect of any one species. The term also helps to capture a major conceptual breakthrough in periodontal research. Periodontal disease isn’t caused, as once thought, by one oral bacterium turned dominant and destructive. In a susceptible person, it’s triggered by a structural imbalance of biofilms within the natural ecology of the microbiome. The new order alters the collective mood of the biofilm and unmasks emergent infectious behavior. Previously benign species within a biofilm opportunistically exploit the imbalance to expand their numbers, overgrow their boundaries, and spread their flagella into the gumline to cause trouble.
Modulating Inflammation: In 1976, institute grantee Roy Page, D.D.S., Ph.D., and his European colleague Hubert Schroeder, D.D.S., published a landmark paper proposing the possible phases of periodontal disease. In 1997, Dr. Page and two of his collaborators revisited the model, likening it this time to four scenes of a play. The opening act introduces the infection of the gumline, and scene two presents a blast of acute inflammation. That’s where the curtain drops for most of us. But for those with periodontitis, the show rolls on to the potentially treacherous scene three, when large numbers of innate and adaptive immune cells — the heavier artillery — infiltrate the gum tissue to stamp out the infection. If all goes well, scene three will fade into a happy ending: resolution of the immune response, sunsetting into the healing of the inflamed tissue.
The Page-Schroeder Model continues to help frame thinking about periodontal disease. It also helps to illustrate a key discovery: Some people with periodontitis seem to get stuck in scene three. Whereas the immune system and its cast of infection-fighting cells dominate the opening of the scene, microbes somewhere out there in the audience make them forget their lines and recite a different script. Instead of clearing the infection, the immune cells get stuck repeating pro-inflammatory signals at levels desired by the microbes. The inflammation slowly degrades the tissues supporting the tooth, giving the microbes a steady food source to fuel their continued growth, though clearly to the detriment of the tooth.
Looking ahead, the research challenge is to ensure that immune cells that stumble over their lines get back on script to make their programmed transitions to resolution and healing. What’s different now than a generation ago is that researchers have begun to work out how these microbes short-circuit the programmed crosstalk of the immune cells. They are also learning to decipher the crosstalk, its programmed ebb and flow, and the full cast of molecular characters engaging in the dialogue. Once these communication codes are cracked, they can be exploited and translated into therapies for the public good.
For example, in recent years, NIDCR researchers established that immune cells known as T helper 17 cells can stimulate the oral inflammatory process, making them a regulatory link between oral bacteria and gum inflammation. More recently, the same group discovered that even non-immune cells, namely the epithelial cells that line the outer layer of the gums, can instigate periodontal inflammation. These researchers also found that a buildup of a protein called fibrin triggers an overactive immune response that damages the gums and underlying bone. Taken together, these discoveries have great potential for developing more
targeted approaches to modulate — and control — the destructive inflammation that drives periodontal disease.
Periodontal Medicine: Since the early 20th century, dentists and researchers have debated the possibility that bacteria from oral infections can enter the bloodstream, colonize vital organs, and wreak havoc.
Through the decades, many researchers have explored the so-called “oral-systemic disease connection.” From the 1980s onward, studies have continuously found statistical associations that, like circumstantial evidence in a court case, implicate periodontitis to some degree in a range of health conditions. The problem has been taking the next evidentiary step and proving direct causation. For many years, scientists lacked the needed research tools to take that step. But that’s now changing, and several recent studies have generated compelling data to show that, at the very least, oral bacteria may contribute to heart disease, diabetes, rheumatoid arthritis, and other conditions. Gaining a more detailed picture of the periodontal disease-systemic disease connection and its causal triggers will be critical in learning to protect human health for a lifetime.
Collaboration Is Key
Periodontal disease researchers have benefitted tremendously through the years from partnering with creative minds in other research disciplines. This collaborative spirit continues today. The collaborative benefits, though, are mutual. Because of the mouth’s easy accessibility and diversity of biology and tissues, it is an ideal part of the body to pilot new ideas. Bathed with saliva, the oral cavity has a unique microbiome that is thought to influence the gut microbiome.
Indeed, it’s easy to envision work on periodontal disease contributing toward the advancement of some of the most active areas of biomedical research. These areas include genomics, proteomics, immunology, microbiology, exposomes, super-resolution imaging, tissue regeneration, big data, artificial intelligence, immunotherapy, microbiome replacement therapy, and precision medicine.
“There is so much to be excited about right now in periodontal research,” said Preethi Chander, Ph.D., director of the institute’s Salivary Biology and Immunology Program, which helps to oversee NIDCR’s periodontal disease research portfolio. “I’m very hopeful that a new generation of treatments will be on the horizon that will expand our clinical capabilities and help people in meaningful ways.”
Here is just a sampling of some recent research progress in the field:
-
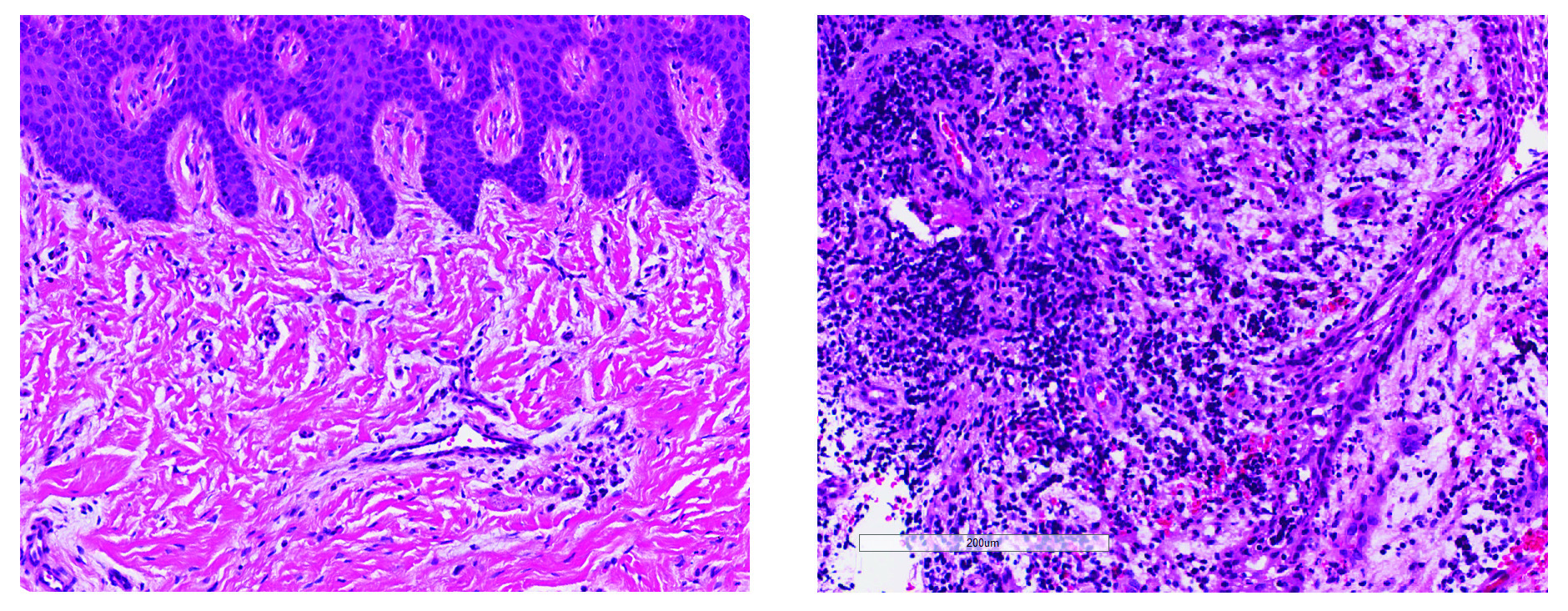
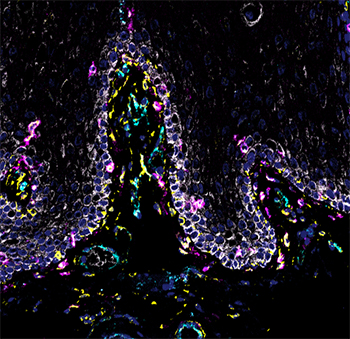
NIDCR scientists and grantees compiled the first human cell atlas of the oral mucosa — the soft pink tissue that lines the inside of the mouth — in healthy and diseased states. The project was no small undertaking. It required performing single-cell analyses of more than 88,000 cells taken from a total of four locations in the human cheek and gumline. The team collected and analyzed the messenger RNA of each cell to record which genes were turned on or off. The on-off record was logged for the various cell types in the different layers of the mucosa, painting a comprehensive genomic picture of how each cell creates its own unique molecular identity in health and inflammatory disease. The atlas now serves as a free, high-quality reference to help other researchers studying periodontal disease.
- Bacteriophages, or “phages” for short, are a prolific band of viruses that selectively (and exclusively) track bacteria to infect and kill them, including those in oral biofilms. This stalking behavior potentially makes them an ideal tool to reshape an unbalanced oral microbiome. The challenge is delivering them where they are most needed. NIDCR grantees recently discovered that the abundant oral bacterium Capnocytophaga gingivalis, with its natural gliding and swarming movements, was well suited to deliver phages to problematic biofilms. The scientists observed that phages were transported over long distances by “surfing” the fluid wake of C. gingivalis swarms. Phages delivered this way penetrated and disrupted previously inaccessible regions of biofilms, suggesting that this transportation method could one day be used to improve therapeutic phage delivery.
- A large phase III clinical trial is being planned to evaluate the effectiveness of an anti-inflammatory treatment for periodontal disease. The treatment, called AMY-101, was developed by an NIH grantee, and NIDCR grantees were actively involved in showing its effectiveness in animal models of periodontitis. AMY-101 inhibits the complement system, a frontline component of our body’s immune defenses. Though the complement system normally does a lot good for a lot of people, some of these plasma proteins are hyperactivated in people with periodontal disease. The extra activity plays a role in transforming the oral microbiome from stable to unbalanced. It also affects the inflammatory response to an invasive microbiome. In a small phase II study, injection of AMY-101 into the gum tissue once weekly for three consecutive weeks was found to be safe and to control inflammation in adults with periodontal disease for up to 90 days.
What’s past is indeed prologue. The data gleaned over the last 75 years has set the stage for a golden age of discovery that’s starting to unfold in periodontal research. Dental clinicians can look forward to the arrival of more biologically driven treatments in their practices. Their greater precision and delivery of tailored care will be empowering for practitioners and patients, including the billions of people around the world concerned about an unusual reddening of their gums.
Related Links:
References:
Tonetti MS, Jepsen S, Jin L, Otomo-Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J Clin Periodontol. 2017 May;44(5):456-462. doi: 10.1111/jcpe.12732. Epub 2017 May 8.
Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976 Mar;34(3):235-49.
Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997 Jun;14:33-53. doi: 10.1111/j.1600-0757.1997.tb00191.x.
Brewer RC, Lanz TV, Hale CR, Sepich-Poore GD, Martino C, Swafford AD, et al. Oral mucosal breaks trigger anti-citrullinated bacterial and human protein antibody responses in rheumatoid arthritis. Sci Transl Med. 2023 Feb 22;15(684):eabq8476. doi: 10.1126/scitranslmed.abq8476. Epub 2023 Feb 22.
Marotz C, Molinsky R, Martino C, Bohn B, Roy S, Rosenbaum M, et al. Early microbial markers of periodontal and cardiometabolic diseases in ORIGINS. NPJ Biofilms Microbiomes. 2022 Apr 20;8(1):30. doi: 10.1038/s41522-022-00289-w.
FOCAL infection. J Am Med Assoc. 1952 Oct 4;150(5):490-1. doi: 10.1001/jama.1952.03680050056016.
Williams DW, Greenwell-Wild T, Brenchley L, Dutzan N, Overmiller A, Sawaya AP, et al. Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell. 2021 Jul 22;184(15):4090-4104.e15. doi: 10.1016/j.cell.2021.05.013. Epub 2021 Jun 14.
Ratheesh NK, Zdimal AM, Calderon CA, Shrivastava A. Bacterial Swarm-Mediated Phage Transportation Disrupts a Biofilm Inherently Protected from Phage Penetration. Microbiol Spectr. 2023 Aug 17;11(4):e0093723. doi: 10.1128/spectrum.00937-23. Epub 2023 Jun 26.
Hasturk H, Hajishengallis G; Forsyth Institute Center for Clinical and Translational Research staff; Lambris JD, Mastellos DC, Yancopoulou D. Phase IIa clinical trial of complement C3 inhibitor AMY-101 in adults with periodontal inflammation. J Clin Invest. 2021 Dec 1;131(23):e152973. doi: 10.1172/JCI152973.
Hasturk H, Schulte F, Martins M, Sherzai H, Floros C, Cugini M, et al. Safety and Preliminary Efficacy of a Novel Host-Modulatory Therapy for Reducing Gingival Inflammation. Front Immunol. 2021 Sep 13;12:704163. doi: 10.3389/fimmu.2021.704163.
Attention Editors
Reprint this article in your own publication or post to your website. NIDCR News articles are not copyrighted. Please acknowledge NIH's National Institute of Dental and Craniofacial Research as the source.
Subscribe for NIDCR Updates
Receive email updates about the latest advances in dental, oral, and craniofacial research.
October 2024